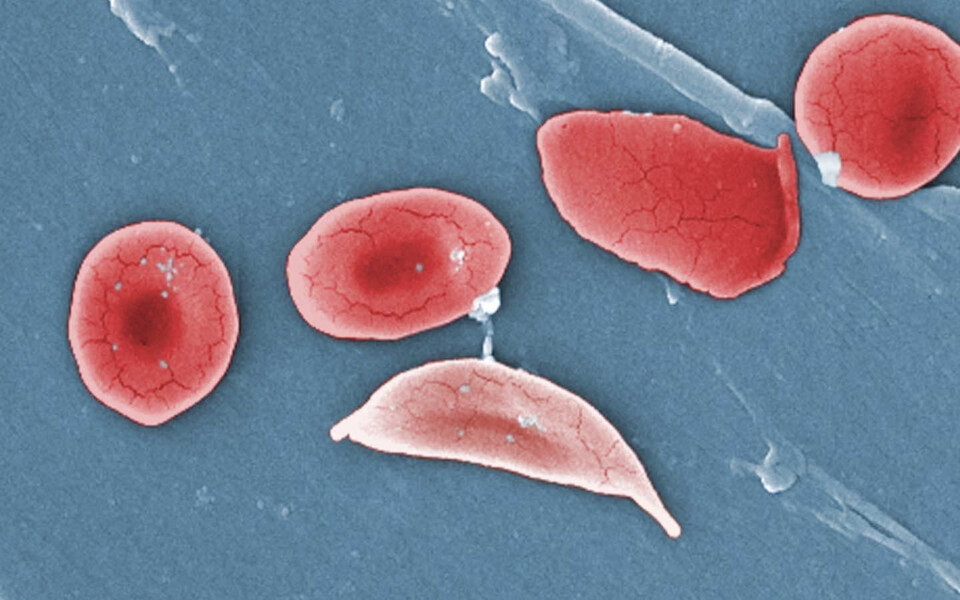
FDA approves two sickle cell therapies, including first CRISPR medicine
One of the new treatments, named Casgevy, is based on CRISPR, a gene-editing tool that moved lightning-fast from a scientific breakthrough in 2012 to a therapy that can alleviate suffering. In the wake of the FDA approval, experts anticipate that treating sickle cell disease will be the first of many medical applications for this technology.
The other treatment, developed by Bluebird Bio and called Lyfgenia, uses a harmless virus to insert a gene into a patient’s stem cells. The treatments are approved for patients 12 and older who experience repeated pain episodes.
“I’ve been taking care of kids with sickle cell for over 30 years, and I’ve been waiting for something like this to happen for a long, long time,” said Lewis Hsu, chief medical officer of the Sickle Cell Disease Association of America and a pediatric hematologist at the University of Illinois at Chicago.
In clinical trials, both therapies freed the overwhelming majority of patients from severe pain crises. Several of the patients who received Casgevy, developed by Vertex Pharmaceuticals and CRISPR Therapeutics, testified before a federal advisory committee in late October, sharing emotional stories about how the therapy opened up their lives, giving them the ability to work or attend school, be present with their families and imagine a future.